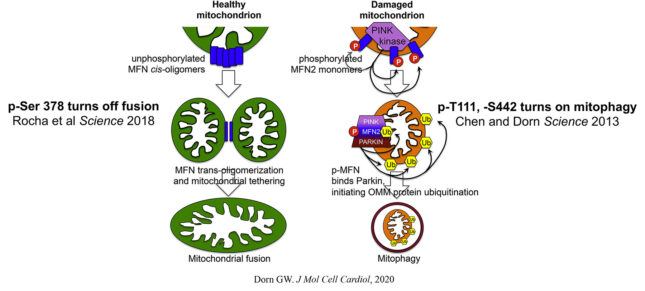
Mechanisms of mitofusin regulation
When we first started studying mitofusins we were surprised at the lack of understanding about what turned these proteins on and off, i.e. how some mitochondria know when to fuse and others know not to. Our first indication was that phosphorylation might regulate mitofusin activity; we discovered that the PINK1 kinase phosphorylated a threonine (T111) and a serine (S442) on MFN2, enabling it to bind Parkin. The functional consequence of these phosphorylation events was to abrogate fusion and accelerate mitophagy. Subsequent studies demonstrated the importance of this “MFN2 phosphorylation switch” in mitophagy-mediated metabolic remodeling of perinatal hearts. Ultimately, we discovered that the biophysical mechanism by which phosphorylation of MFN2 promotes alternate functioning was a conformational change that alters protein pairing. This paradigm prompting our development of allosteric small molecular mitofusin activators now undergoing preclinical evaluation for diseases of impaired mitofusin function. The laboratory continues to interrogate the consequences of post-translational MFN regulation by phosphorylation and other means, extending our functional endpoints to include mitochondrial motility.
Relevant citations:
- Chen Y, Dorn GW II. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. Apr 26;340(6131):471-5, 2013. PubMed PMID: 23620051; PubMed Central PMCID: PMC3774525.
- Gong G, Song M, Csordas G, Kelly DP, Matkovich SJ, Dorn GW II. Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science, 350:aad2459, 2015. PubMed PMID : 26785495; PubMed Central PMCID: PMC4747105.
- Franco A, Kitsis RN, Fleischer JA, Gavathiotis E, Kornfeld OS, Gong G, Biris N, Benz A, Qvit N, Donnelly SK, Chen Y, Mennerick S, Hodgson L, Mochly-Rosen D, Dorn GW II. Correcting mitochondrial fusion by manipulating mitofusin conformations. Nature 540:74-79, 2016.
Related news release:
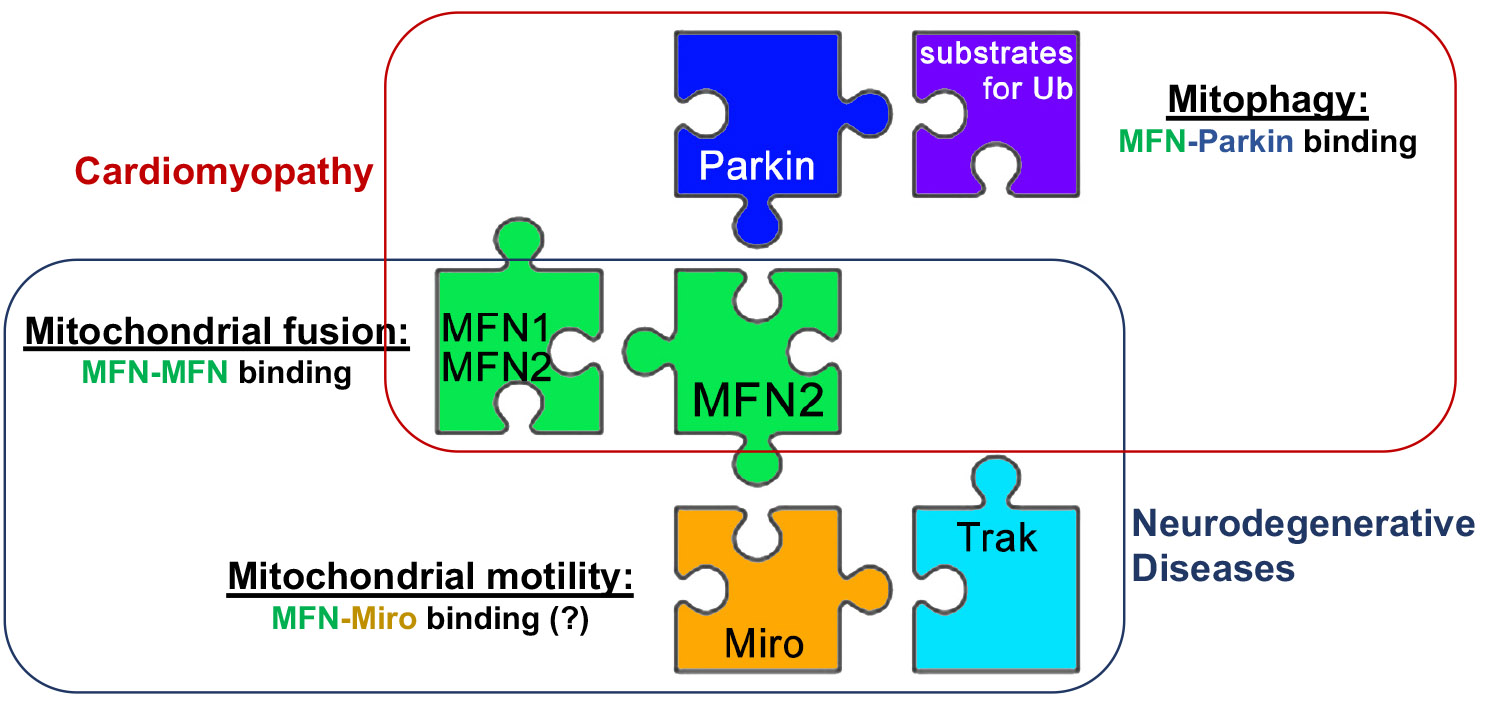
The mitochondrial dynamics-mitophagy interactome in heart disease
The discovery that phosphorylation of MFN2 by the mitochondrial-localized and damage-stabilized kinase PINK1 transformed it from a fusion factor into a mitochondrial receptor for the mitophagy factor, Parkin, proved to be fertile ground for our efforts to understand the inter-relationships between mitochondrial dynamism, mitochondrial biogenesis, and mitophagic mitochondrial quality control in hearts. Our studies revealed how these processes, far from being functionally distinct, are connected through molecular cross-talk, duplication and multiplicity of function, and counter-regulation. This work evolved into an ongoing detailed mechanistic evaluation of the role of PINK1-Mfn2-Parkin mitophagy signaling in cardiac metabolic remodeling after hemodynamic or ischemic stress.
Relevant citations:
- Song M, Mihara K, Chen Y, Scorrano L, Dorn GW II. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. Feb 3;21(2):273-85, 2015. PubMed PMID: 25600785; PubMed Central PMCID: PMC4318753.
- Song M, Franco A, Fleischer JA, Zhang L, Dorn GW II. Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab. 26:872-885, 2017. PubMed PMID: 29107503; PubMed Central PMCID: PMC5718956.
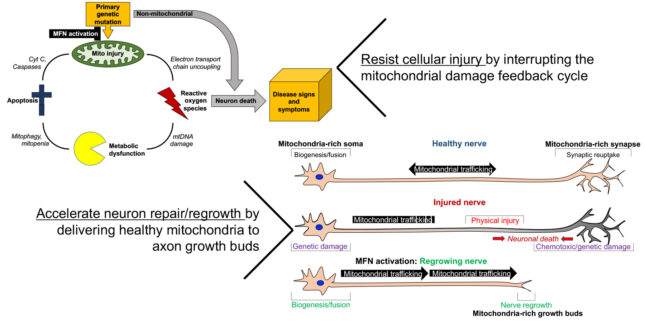
Correcting mitofusin dysfunction in neurodegenerative disease
Our laboratory conceived an unconventional notion of how different mitofusin conformations might facilitate or impede mitochondrial tethering, fusion and transport. We identified intra- and intermolecular protein interactions that control conformational shifts, and developed first in class mitofusin activators. Some additional medicinal chemistry was required to produce drug-like compounds useful for in vivo studies in mouse disease models. The prototype mitofusin activator rescued in vitro and in vivo models of Charcot Marie Tooth disease type 2A cause by loss-of-function Mfn2 mutations. Ongoing research projects are using pharmacological mitofusin activation to mitigate neuronal degeneration in other peripheral and central neuropathies.
Relevant citations:
- Rocha AG, Franco A, Krezel AM, Rumsey JM, Alberti JM, Knoght WC, Biris N, Zacharioudakis E, Janetka JW, Baloh RH, Kitsis RN, Mochly-Rosen D, Townsend RR, Gavathiotis E, Dorn GW II. Mfn2 agonists reverse mitochondrial defects in preclinical models of Charcot Marie Tooth disease type 2A. Science, 360:336-341, 2018.
- Dang X, Zhang L, Franco A, Li J, Rocha AG, Devanathan S, Dolle RE, Bernstein PR, Dorn GW II. Discovery of 6-phenylhexanamide derivatives as potent stereoselective mitofusin activators for the treatment of mitochondrial diseases. J Med Chem, 63:7033-51, 2020.
- Franco A, Dang X, Walton EK, Ho JN, Zablocka B, Ly C, Miller TM, Baloh RH, Shy M, Yoo AS, Dorn GW II. Burst mitofusin activation reverses neuromuscular dysfunction in murine CMT2A. Elife, 9:e61119, 2020.
- Dang X, Williams SB, Devanathan S, Franco A, Fu L, Bernstein PR, Walters D, Dorn GW II. Pharmacophore-Based Design of Phenyl-[hydroxycyclohexyl] Cycloalkyl-Carboxamide Mitofusin Activators with Improved Neuronal Activity. J Med Chem. 64:12506-12524, 2021.
Related news releases: